Jul - 23 - 2013
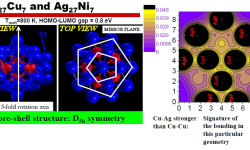
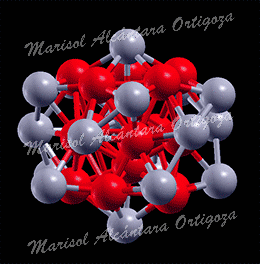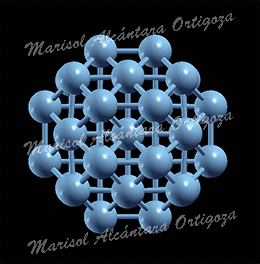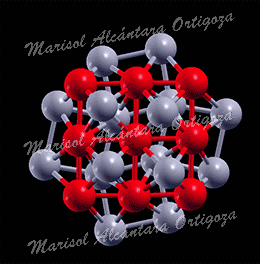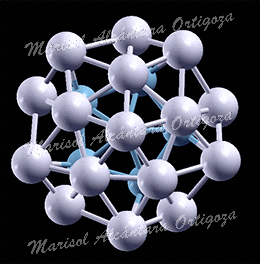
[Phys. Rev. B 77, 195404 (2008) and Contemporary Physics: Proceedings of the International Symposium by Jamil Aslam, Faheem Hussain, Riazuddin; Published by World Scientific (2008)] It is quite clear now that small nanoclusters necessarily have physical and chemical attributes that are distinct from those of their pure bulk counterparts, features that are of course attractive to achieve specific catalytic and optical properties. Needless to say, bimetallic nanoparticles broaden the range of applications, and […]
Jul - 23 - 2013
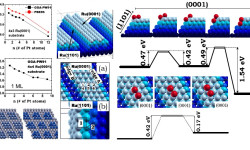
[Phys. Rev. B 78, 195417 (2008) and J. Phys.: Condens. Matter 21, 474226 (2009)] This work focuses on understanding the performance of clean energy conversion nanodevices, proton exchange membrane fuel cells (PEMFCs) in particular. Operation of the anode in these devices consists of splitting hydrogen molecules into protons and electrons and Pt atoms on the anode catalyze such process. Unfortunately, the main source of hydrogen is hydrocarbons, which unavoidably contain carbon monoxide (CO) molecules. […]
Jul - 23 - 2013
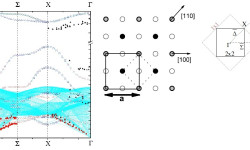
[Phys. Rev. B 81, 115465 (2010)] Controlling and exploiting self-ordering of materials requires us to understand the structure at the atomic level and the mechanism that drives the nanoscale self-assembling. The result of adsorbing nitrogen on the Cu(001) surface constitutes one example of nanoscale self-ordering as it forms squared 6×6 nm2 N-patches on top of the Cu surface. Such structure of N-patches and clean Cu channels is a prototype of templates […]
May - 29 - 2013
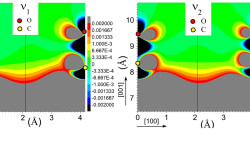
[J. Phys. Chem. A, 115 (25), 7291 (2011)] The coupling between molecules and metallic surfaces has attracted much interest in the last three decades because of the insight it provides into chemisorption and diffusion processes. The interactions present when carbon monoxide (CO) is absorbed on metals surfaces are of particular importance since they influence chemical reactions of utmost technological importance and now even data storage. Only recently, because of the advances in […]
May - 29 - 2013
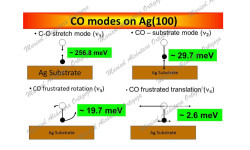
This work follows in many ways that performed for the adsorption of CO on Cu(001) [1]. Yet, it opened a wealth of new insights about the way we characterize CO adsorption. The interest on CO adsorption on Ag surfaces lies on the ability of Ag(110) to catalyze the formation of CO2 and CO3 from O2 and CO reactants at temperatures low enough (~100 K) to preserve oxygen in its molecular […]