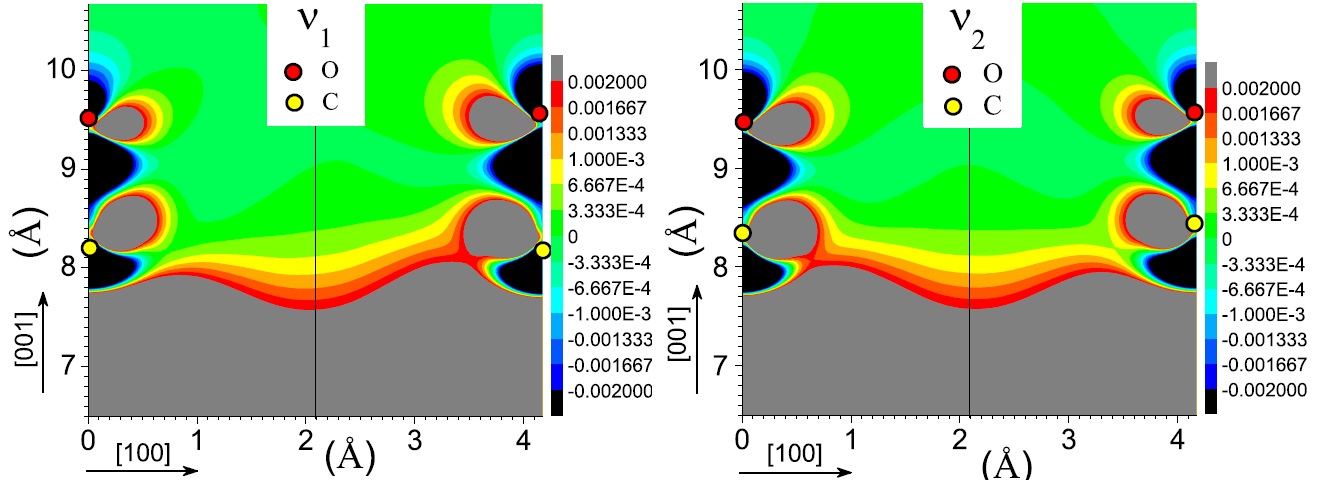
[J. Phys. Chem. A, 115 (25), 7291 (2011)] The coupling between molecules and metallic surfaces has attracted much interest in the last three decades because of the insight it provides into chemisorption and diffusion processes. The interactions present when carbon monoxide (CO) is absorbed on metals surfaces are of particular importance since they influence chemical reactions of utmost technological importance and now even data storage. Only recently, because of the advances in computational accuracy and power, in a set of four articles [1-4], we have revealed the nature of bond between CO and silver, the existence of strong CO-CO interactions even at relatively long distances, the nature of such interactions, as well as the fact that these do influence their diffusion through a combined analysis of the structure as well as the electronic and vibrational properties of CO on noble metal surfaces. First of all, measurements and our calculations of the vibrational properties – that uniquely fingerprint structure and test stability- for the first time confirmed with remarkable confidence the structure predicted for these CO-metal systems [1-3]. Furthermore, based on our calculations, we have reinterpreted experimental data essential to characterize both the CO-metal coupling and even the experimental probe to characterize the system [3]. Our electronic structure calculations have also shown that the interaction between carbon monoxide and noble metal surfaces is mostly of a chemical nature, through charge donation and back-donation between CO and the metal substrate, even for silver. This is a remarkable result since, in the case of Ag, the adsorption of CO had long been characterized as physisorption [1,2,4]. Besides, we have also shown that the simple criterion followed to gauge the strength of the CO-metal bond, based on two vibrational frequencies, can be misleading and explained the apparently anomalous vibrational frequencies of CO on Ag(001). Furthermore analysis of the frustrated translation mode of CO on Cu and Ag metal substrates demonstrates that CO diffusion is influenced by CO-CO interactions. More importantly, an analysis of the C-O stretch mode and of the charge density as the vibration takes place revealed the reason for which, even at relatively long distances, CO-CO interactions are still present and significant. I have shown that the main origin of intermolecular interactions is not electrodynamical in nature but chemical. Namely, we have shown that the C-O vibration creates fluctuations in the charge exchanged between CO and the metal and thus that charge fluctuation occurring in the metal mediates the interactions between molecules.
References
[1] Effect of c(2×2)-CO overlayer on the phonons of Cu(001): A first-principles study
[2] Ab initio Calculations of the Dispersion of Surface Phonons of a c(2×2)-CO overlayer on Ag(001)
[3] Effect of c(2×2)-CO overlayer on the phonons of Cu(001): A first-principles study