[Phys. Rev. B 77, 195404 (2008) and Contemporary Physics: Proceedings of the International Symposium by Jamil Aslam, Faheem Hussain, Riazuddin; Published by World Scientific (2008)] It is quite clear now that small nanoclusters necessarily have physical and chemical attributes that are distinct from those of their pure bulk counterparts, features that are of course attractive to achieve specific catalytic and optical properties. Needless to say, bimetallic nanoparticles broaden the range of applications, and even more so as we turn to the so-called core-shell nanoparticles that contrast to “nanoalloys”.
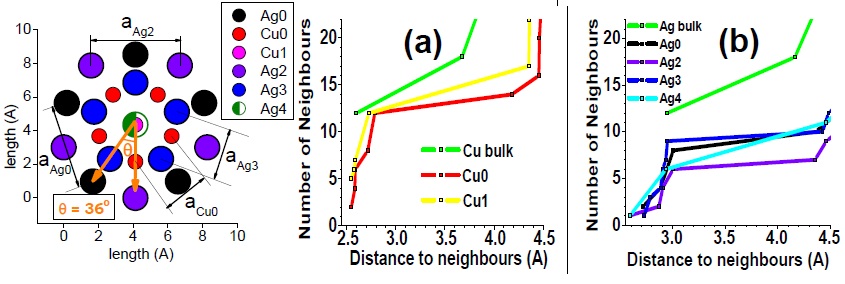
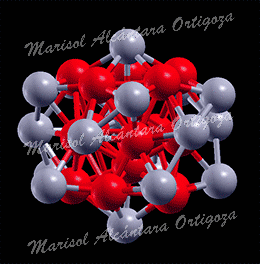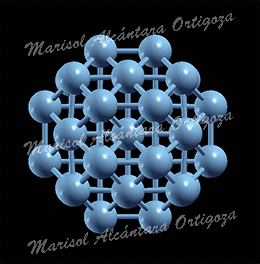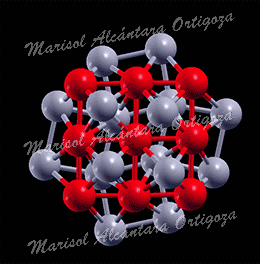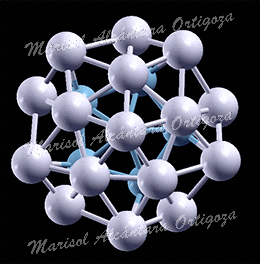
(Left) Top view of Ag27Cu7 nanocluster, perpendicular to the mirror plane. (Right) Bond coordination for Cu and Ag atoms of Ag27Cu7 compared with Cu and Ag bulk, respectively. Figure taken from Phys. Rev. B 77, 195404 (2008)
Synthetizing and characterizing nanoparticles still demands significant efforts. From the fundamental point of view, for example, characterizing the structure of a small nanoparticle continues to be a challenge both theoretically and experimentally. From the practical point of view, on the other hand, some issues such as chemical stability relative to other reagents and to cluster assembling (sintering) represent difficulties for technological applications, including those in catalysis and the formation of the so-called cluster crystals.
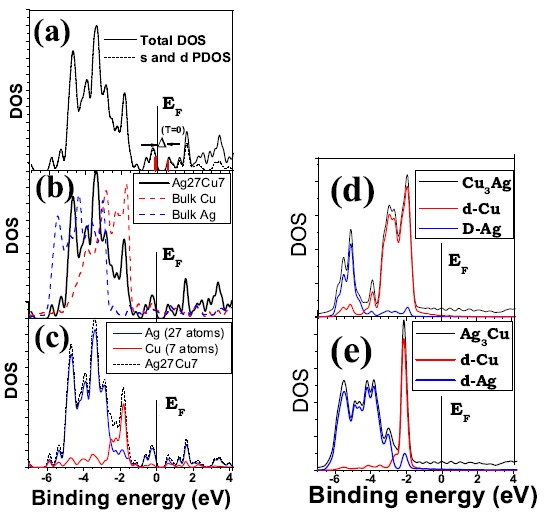
(a) Total and projected electronic DOS of Ag27Cu7. The later corresponds to the contribution of s and d atomic states between -7 and 4.2 eV from the Fermi level, EF , which is set equal to 0. The s-contribution is negligible up to EF . The HOMO-LUMO gap in the ground state, 0.77 eV, is highlighted in red; (b) comparison between the total DOS of Ag27Cu7 and that of Ag and Cu bulk; (c) contribution from the core (Cu) and shell (Ag) atoms to the projected DOS of Ag27Cu7; (d) DOS of Cu3Ag showing the d-contribution from each species to the total DOS of the alloy in the L12 phase; (e) DOS of Ag3Cu showing the d-contribution from each species the total DOS of the alloy in the L12 phase. Figure taken from Phys. Rev. B 77, 195404 (2008)
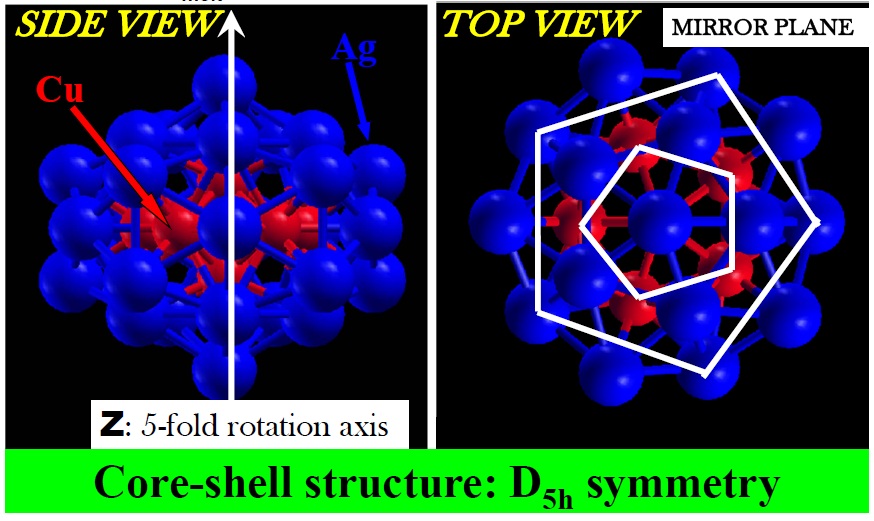
I have made a step forward in this direction in reference [1] by revealing the factors that come into play for stabilizing core-shell nanoparticles. In earlier investigations, it was found that among the Ag-Cu family of 34-atom nanoparticles, Ag34-xCux, Ag27Cu7 is the composition with lowest heat of formation and is attained via a particular core-shell structure. This finding and the method used to discover it are interesting by themselves yet, from the fundamental point of view, left open several important questions: why is this composition more stable that the others? Why would it be stable if in the bulk Ag and Cu segregate at all compositions?
By analyzing the composition, structure and electronic properties of Ag27Cu7, I have revealed that the stability of Ag27Cu7 is the result of optimizing the maximum number of Cu-Cu and Cu-Ag bonds, using the minimum number of Cu atoms. Regarding segregation tendencies of Ag and Cu, I objected that core-shell Ag-Cu nanoalloys behave “differently” from the corresponding bulk alloys, as pointed by other authors. This is not the case since Ag27Cu7 is evidently segregated by construction. In fact, the segregated structure is the attribute that leads to its relative stability, provided the core-shell structure allows formation of strong Cu-Cu and Cu-Ag bonds without contracting the typical and relatively long Ag-Ag bonds and (most importantly) without stretching the typical and relatively short Cu-Cu bond.

2D Charge density plot at a plane that contains the 5-fold rotation axis of Ag27Cu7 nanoalloy and is, therefore, perpendicular to its mirror plane. Atoms labelled with black color are precisely centered on that plane.
Although I showed that this particular structure is able to render Cu-Ag bonds slightly stronger than its Cu-Cu bonds (a feature not recognized before), I have inferred that there exists a hierarchy of bond strength intrinsic only to the constituting species, Cu and Ag, for example [Cu-Cu > Cu-Ag > Ag-Ag]. Clearly, the strength and length of any bonds are correlated properties in any particular structure. Therefore, nanostructures that enforce such a hierarchy must also obey a bond-length order [Ag-Ag> Cu-Ag> Cu-Cu]. This is an important result because such hierarchies are not particular to Ag and Cu and can be applied to understand and design any bimetallic material.
References:
[1] M. Alcántara Ortigoza and T. S. Rahman; “First principles calculations of the electronic and geometric structure of Ag27Cu7 nanoalloy“; Phys. Rev. B 77, 195404 (2008)
[2] M. Alcántara Ortigoza and T. S. Rahman; “Symmetry and novelty in the electronic and geometric structure of nanoalloys: the case of Ag27Cu7“; Contemporary Physics: Proceedings of the International Symposium by Jamil Aslam, Faheem Hussain, Riazuddin; Published by World Scientific (2008)